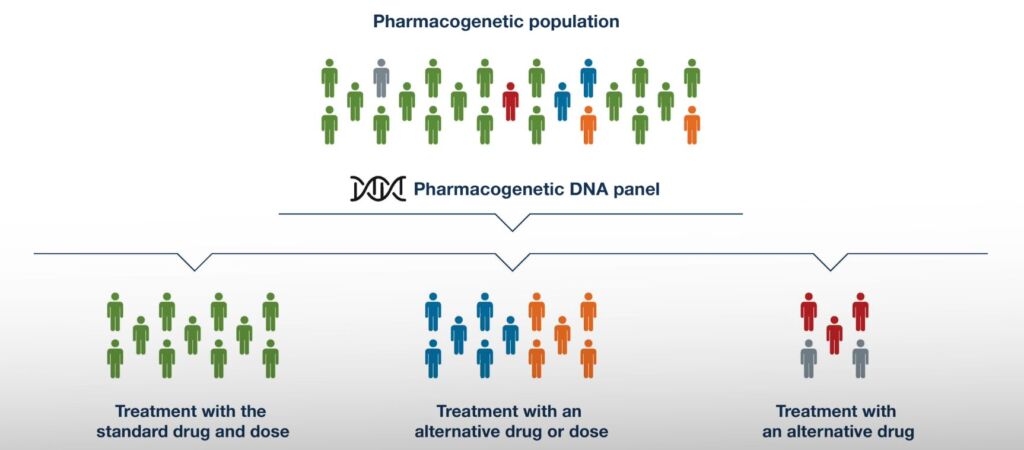
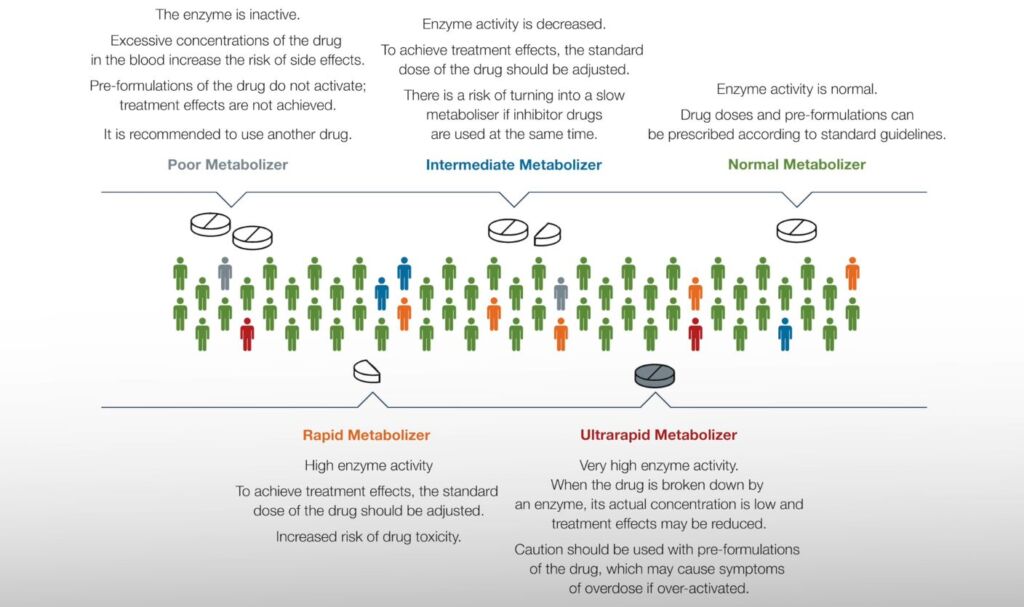
One medicine at the same dose does not suit everyone equally – an effective dose for some may not produce therapeutic effects for others, may cause serious side effects, or even be life-threatening. Up to 20% of ambulatory patients experience adverse reactions to medications, and 10-20% of hospitalized patients experience at least one adverse reaction during hospitalization.
Pharmacogenetics DNA panel enables the investigation of a patient’s personalized genetic profile, allowing appropriate medications to be determined at the right dosage, thereby increasing the likelihood of successful treatment while reducing the risk of dangerous side effects or ineffective treatment.
- Defining genetic variants influencing the effects of drugs in the organism and elucidating the relationships between genes and drug responses.
- Preventing serious drug side effects and drug interactions.
- Developing an optimal treatment plan based on the patient’s unique genetic information.
- Replacing common drug choices and trial-and-error treatment plans.
- Reducing overall healthcare costs.
- Conducting simultaneous examination of multiple genes is faster and more cost-effective than studying individual genes separately.
1. Knowing the patient’s pharmacogenetic profile allows the physician to promptly and specifically prescribe the appropriate medication at a customized dosage.
Patient response to treatment should be considered across all medical specialties. The use of pharmacogenetic analyses is particularly valuable for the following medications:
- Antidepressants and other psychotropic drugs;
- Analgesics/rheumatoid drugs;
- Anticoagulants;
- Antibiotics, antiviral, and antifungal drugs;
- Diabetes medications;
- Antihypertensive drugs;
- Cytostatics;
- Proton pump inhibitors.
2. The quality of treatment improves, and treatment costs decrease.
3. Among pharmacogenetically tested patients, the following are observed:
- Greater likelihood of symptom relief compared to conventional treatment;
- Significantly better rates of treatment response and tolerability;
- Shorter hospitalization time;
- Lower treatment costs.
4. Among pharmacogenetically tested patients who concurrently use multiple medications, the following are observed:
- Reduced need for hospitalization;
- Reduced doctor visits.
The panel comprises 29 genes associated with the metabolism of over 200 drug compounds.
| Gene | Function |
| ABCB1 | Codes for P-glycoprotein, which is an essential cellular membrane transport protein. P-glycoprotein controls the entry of compounds into the cell throughout the body, thereby affecting drug concentrations. |
| ABCB2 | Codes for a cell membrane protein that transports several molecules, including drugs, across the membrane. ABCG2 partially transports the same drugs as P-glycoprotein. |
| ALDH2 | Codes for mitochondrial aldehyde dehydrogenase, which oxidizes aldehydes into their corresponding carboxylic acids. |
| BCHE | Codes for nonspecific butyrylcholinesterase, which hydrolyzes various choline-based esters. |
| CACNA1S | Codes for the dihydropyridine receptor alpha1S subunit, expressed in the sarcoplasmic reticulum of muscle cells, activating the RYR1 calcium channel membrane during depolarization in contractile myocytes. |
| CYP1A2 | Hepatic enzyme involved in the metabolism of several drugs, caffeine, and procarcinogens. |
| CYP2B6 | Hepatic enzyme responsible for the metabolism of HIV and cancer drugs, as well as bupropion. |
| CYP2C rs12777823 |
Genetic variant associated with lower doses of warfarin. |
| CYP2C19 | Hepatic enzyme mediating the metabolism of various important drugs, including psychotropic drugs, proton pump inhibitors, and anticoagulants. |
| CYP2C8 | Hepatic enzyme involved in the metabolism of several drugs, including antidiabetic drugs, statins, analgesics, and cancer drugs. |
| CYP2C9 | Hepatic enzyme involved in the metabolism of several drugs, including warfarin and phenytoin. |
| CYP2D6 | Hepatic enzyme mediating the metabolism of approximately 20-25% of drugs in use, including antidepressants, antipsychotics, and analgesics. |
| CYP3A4 | Hepatic enzyme mediating the metabolism of 30-50% of drugs in use. |
| CYP3A5 | Hepatic enzyme involved in the metabolism of several drugs, with tacrolimus being particularly significant. |
| CYP4F2 | Mediates the metabolism of various endogenous substrates and xenobiotics. Genotype information may be useful, for example, in predicting warfarin dosage. |
| DPYD | Codes for dihydropyrimidine dehydrogenase, which catabolizes fluoropyrimidines used as chemotherapeutics for various types of cancer. |
| F2 | Codes for prothrombin, a key enzyme in the coagulation cascade. Mutations in the prothrombin gene predispose to thrombosis. |
| F5 | Codes for coagulation factor V. Mutation in the F5 gene (known as the Leiden mutation) is the most common hereditary mutation causing thrombosis. |
| G6PD | Codes for glucose-6-phosphate dehydrogenase, which protects red blood cells from oxidative stress. |
| GRIK4 | Codes for the kainate receptor (a subtype of glutamate receptor), contributing to glutamatergic signaling. |
| IFNL3 | Codes for interferon lambda 3, triggered during viral infections. Variants of this gene help assess the effectiveness of hepatitis C treatment. |
| MTHFR | Codes for methylenetetrahydrofolate reductase enzyme, critical in folate metabolism, influencing methylation and DNA synthesis pathways. |
| NAT2 | Acetylates and neutralizes various xenobiotics. It partially activates and generates certain carcinogens, the activity of which may be associated with cancer risk (e.g., prostate or colorectal cancer). |
| NFIB | Codes for a transcription factor expressed in various tissues, located on the short arm of chromosome 9. Copy number variants in this region lead to the development of MACID syndrome (macrocephaly and intellectual developmental disorders), and intragenic variants of this gene have been associated with clozapine metabolism. |
| NUDT15 | Codes for nudix hydrolase enzyme, which converts metabolites of thiopurine drugs into less cytotoxic forms. |
| SLCO1B1 | Codes for OATP1B1 protein, facilitating the uptake of statins from plasma into the liver. |
| TPMT | Codes for thiopurine S-methyltransferase, responsible for the metabolism of thiopurine drugs. |
| UGT1A1 | Codes for UDP-glucuronosyltransferase 1-1 enzyme, responsible for the metabolism of certain drugs (e.g., anticancer drugs) and bilirubin elimination. |
| VKORC1 | Involved in the activation of coagulation factors and has hereditary forms that directly affect warfarin dosing. |
Pharmacogenetic analysis does not require specific pre-analytics or special sampling procedures. During the routine procedure of venous blood collection, the patient donates a sample. Oral mucosal swab is also a suitable material for testing. The sample is then transported to the laboratory, where the analysis begins.
As result of that, a detailed report is generated, which includes not only gene-specific results but also a comprehensive overview of medications with dosage recommendations, as well as a summary of the tested genes and predicted phenotypes. The report can be viewed in Estonian, English, Finnish, French, German, Swedish, and Dutch.
Sampling → DNA extraction → OpenArray and CNV analysis → Interpretation
Genotype raw data is organized by genes. Several medical specialists are responsible for the accuracy and currency of the information. Report includes:
The genes included in the panel are associated with the metabolism of over 200 drug compounds, which are related to various therapeutic areas.
Medications are grouped based on the results:
- Medications with clinically significant genetic variations;
- Medications with certain clinical significance genetic variations;
- Medications with low clinical significance genetic variations;
- Medications with no clinically significant genetic variations.
The report also separately lists heavily affected medications across different therapeutic areas along with their active ingredients and phenotypes.
For each medication, there are drug-specific dosage recommendations based on scientific databases (such as CPIC, etc.). The recommendations are classified according to the safety of the medication:
![]() Pharmacogenetic variation affects drug effectiveness or adverse reactions with significant clinical relevance. A genetic test is
Pharmacogenetic variation affects drug effectiveness or adverse reactions with significant clinical relevance. A genetic test is
recommended. Check existing test results before prescribing the drug. Check dosing and administration based on test results.
![]() Pharmacogenetic variation affects drug effectiveness or adverse reactions with some clinical relevance. If genetic test results are
Pharmacogenetic variation affects drug effectiveness or adverse reactions with some clinical relevance. If genetic test results are
available, consider changing drug or dosing based on results. If genetic testing has not been conducted, consider ordering a test.
Pharmacogenetic variation may affect drug effectiveness or adverse reactions, but with minor clinical significance in most
patients. Monitor drug response and possible adverse reactions. If genetic test results are available, consider changing drug or
dosing based on results.
Pharmacogenetic variation does not significantly affect drug effectiveness or adverse reactions.
Pharmacogenetics DNA panel is indicated in the following cases:
- Initiation of drug therapy;
- Determination of the appropriate dosage;
- Suspected drug effectiveness;
- Prevention of drug side effects.
Sample material: EDTA-blood, oral mucosal swab
Storage of the sample: at room temperature for 24 hours; 2-8 °C for 5 days; longer if frozen.
Turnaround time (TAT) for results: 21 business days
Price: 315 €
The Pharmacogenetic DNA panel can be ordered:
- By a doctor for the patient electronically from the doctor's program or through a paper referral
An electronic referral can be generated from the doctor’s program, which has previously established integration with SYNLAB through the Medipost data exchange platform. Currently, active interfaces exist with the programs Perearst2, Perearst3, LIISA, eKliinik, Watson/Winston, Ester/Heda, MIS.
The analysis can be found in SYNLAB’s list of research groups under the name “Farmakogeneetika DNA paneel” within the research group „Pärilike haiguste, riskialleelide uuringud“:
- Double-click on the analysis.
- Enter the barcode number and date and time in the appropriate fields.
- Depending on the program, press “Save” and “Send” or “Submit”.
The barcode entered into the program must be vertically affixed to the sample label. If your institution does not use barcodes, please enter the date and time into the doctor’s program and print out the referral directly from the program. In this case, mark the sample tube with the patient’s name as indicated on the referral.
If electronic ordering is not available, a paper referral can be issued (either a computer-fillable version or a printable version). The referral must include the patient’s and requester’s information, as well as the date and time of sample collection. From the list of analyses, mark “Farmakogeneetika DNA paneel.”
The logistics of sample collection to SYNLAB’s molecular biology laboratory in Tallinn are organized by SYNLAB. If sample collection does not take place at your institution, patients can be directed to SYNLAB’s sample collection sites. Reservations for blood collection are not necessary; patients are served on a first-come, first-served basis. It is important that the order is previously entered into the doctor’s program or a referral is provided to the patient.
Once the analysis is completed, the invoice will be sent to the referring clinic. If you wish for the patient to pay for the analysis at the laboratory, please use a self-payer referral.
- As a private customer in Estonia from My SYNLAB
- If you are interested in ordering Pharmacogenetic DNA panel outside Estonia, please contact us via e-mail icsales@synlab.ee
- Pharmacogenetics: The right drug for you | Nature
- Meta-analysis of probability estimates of worldwide variation of CYP2D6 and CYP2C19 | Translational Psychiatry (nature.com)
- Pharmacogenetic testing among patients with mood and anxiety disorders is associated with decreased utilization and cost: A propensity‐score matched study – Perlis – 2018 – Depression and Anxiety – Wiley Online Library
- Incidence, preventability, and causality of adverse drug reactions at a university hospital emergency department | SpringerLink
- https://www.tai.ee/et/personaalmeditsiini-uudiskirjad/uuringud-farmakogeneetiline-testimine-aitab-ravi-tohusamaks-muuta
- Using pharmacogenetic testing in psychiatry, Jari Forsström, Chief Medical Officer, Abomics Oy